| STRUCTURE OF THE MOST
SUPERFICIAL LAYER OF ARTICULAR CARTILAGE
R. TESHIMA, T. OTSUKA, N. TAKASU, N.
YAMAGATA, K. YAMAMOTO
Tottori University, Yonago, Japan
We studied the most superficial layer of macroscopically
normal articular cartilage obtained from human femoral heads,
using polarising microscopy and SEM. The most superficial
layer, 4 to 8 my thick, was acellular consisting of collagen
fibrils. This layer could be peeled away as a thin film,
with no broken collagen fibrils on its inferior surface or
on the surface of subjacent cartilage layers. The orientation
and diameter of collagen fibrils were different on these two
surfaces. Our findings suggest that the most superficial layer
is an independent one which is only loosely connected to the
fibrous structure in the layer deep to it.
J Bone Joint Surg [Br] 1995;77-B:460-4.
Receved 24 June 1994.. Accepted after revision 20 October
1994
The superficial structure of articular cartilage has been
frequently studied since the 18th century, when John Hunter
noted a membrane on the cartilage with a magnifying lens (Ghadially
1983), but there is no clear consensus about this structure.
We examined the most superficial layers of human femoral-head
articular cartilage, using polarising microscopy and SEM.
MATERIALS AND METHODS
We collected 20 human femoral heads removed at arthro plasty
for fracture of the femoral neck or from hips disarticulated
after severe trauma. Nine were discarded because of visible
pathological changes on the articular surface, such as fine
fibrillation or lack of a glossy appearance. Eleven heads
with macroscopically smooth and Glossy surfaces and no apparent
osteoarthritic changes were selected for study from patients
whose ages ranged from 40 to 72 years. Several different
investigations were performed:
1) Four femoral heads were fixed in 10% formalin in phosphate-buffered
solution at pH 7.0, decalcified in phosphate-buffered
10% ethylene diamine tetra-acetic acid at pH 7.1, then dehydrated
and embedded. Frontal plane sections, 10 gm in thickness,
were studied, unstained, by polarising microscopy.
2) From four other femoral heads, strips of cartilage 1.5
x 0.5 cm in size were excised with the subchondral bone. These
were fixed in 2.5% glutaraldehyde in phosphate-buffered
solution at pH 7.0. postfixed in buffered 1 % osmium tetraoxide
for two hours, and dehydrated. They were then freeze-cracked
in a direction perpendicular to the Ion. axis of the strips. The
specimens were dried by the critical point method, and the
cracked surface was sputter-coated with gold and examined
by SEM (Model HFS-2ST; Hitachi, Tokyo, Japan).
3) Immediately after the three remaining femoral heads
had been excised, two slits about 5 mm apart were cut in the
head surface in longitudinal and circumferential directions
with a scalpel. A third slit was made at the base of
these. The superficial edge of the isolated cartilage was
held with small rongeurs and separated by peeling it away. The
separated layer and the cartilage deep to it were prepared
for light microscopy as described above, and 7 my sections
vere cut perpendicular to the articular surface in a longitudinal
direction. The unstained specimens were examined by polarising
microscopy. Parts of the two specinens were processed
as described above for SEM; the deep surface of the superficial
layer and the superficial surface of he deep layer were studied.
4) In a similar fashion, two slits were made from the ;ynovium
of the femoral neck into the articular cartilage in i longitudinal
direction. The synovium was held by small rongeurs and
peeled away towards the articular cartilage. The separated
specimens were examined unstained, and also stained with safranin
0 fast green for light microscopy.
5) The strip specimens of cartilage including subchondral
bone were fixed in 10% neutral formalin for two days, then
macerated with 10% sodium hydroxide at 37gr.C for 24 hours. Some
strips were then dehydrated, embedded and cut into 7 my sections
perpendicular to the articular surface.
6) Using other macerated strips an attempt was made
to prepare specimens similar to those obtained in the surface
separation experiment, but the tissues were more friable and
only relatively small areas of the superficial layer could
be peeled off. The surfaces were then rinsed in phosphate
buffer, postfixed in buffered 1% osmium tetroxide for two
hours, dehydrated and dried by the critical point method. After
sputter-coating, with gold they were examined by SEM.
RESULTS
Polarising microscopy. Polarising, microscopy revealed
a membrane-like structure with strong birefringence over the
entire surface of the articular cartilage. This structure
was 4 to 6 my thick, acellular, and had separated from a deeper
layer consisting of spindle chondrocytes.
Scanning electron microscopy of cracked surfaces. The
cracked surface of the articular cartilage, when examined
by SEM, showed a layer of dense collagen fibrils as the most
superficial layer (Fig. 1). The collagen fibrils ran
parallel to the articular surface, and the thickness of the
layer was 4 to 8 my.
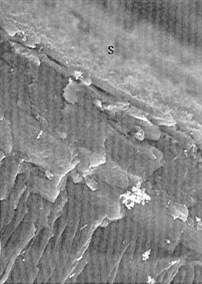

Figure 1 a - SEM of the cracked surface of human articular
cartilage ( x 450). The most superficial structure is
distinctly different from the layer deep to it, and its narrow
edge is seen below the letter S.
Figure 1 b - High-magnification SEM of the sliperficial layer
( x 9000). It consists of a layer of densely-packed collagen
fibrils running parallel to the articular surface.
Surface separation. The superficial layer of articular
cartilage could be separated only in a longitudinal direction. A
white translucent membrane-like structure 0.5 to 1.0 cm in
length was obtained, but it was impossible to obtain more
tissue by circumferential separation. The morphological
structure of this layer was identical to that seen in non-separated
specimens, as were the optical characteristics on polarising
microscopy.
Both the deep surface of the separated layer and the opposing
surface of the remaining, cartilage were composed of collagen
fibrils, but there were distinct differences in the direction
and diameter of fibrils and in the amount of granular material
adhering to them (Figs 2 and 3). The orientation of the
fibrils was clearly seen on the deep surface of the separated
layer: most were orientated in a longitudinal direction on
the femoral head (Fig. 2a). The fibrils on the deep surface
of the superficial layer were 75 to 100 nm in diameter -and
showed no adherent granular substance, while those on the
surface were 125 to 150 nm in diameter and showed a large
amount of an adherent granular substance (Fig. 3). No
broken fibrils were observed on either of the originally opposed
surfaces.
Specimens obtained in continuity with the synovial tissue
of the femoral neck had a white translucent membranelike
structure, similar in appearance to that from the surface
separation experiment. No specimen obtained by separation
from the synovial tissue showed any adherence of cartilage
matrix staining red by safranin 0, but a thin layer of chondrocytes
was adherent to the part near the synovium.
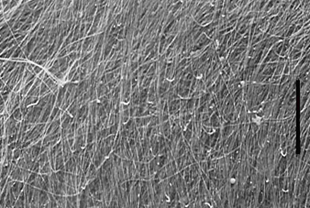
Figure 2a - SEM of the deep surface of the separated superficial
layer ( x 2200). It is composed of collagen fibrils,
most of which run in a radial direction (indicated by the
long bar) on the femoral head.

Figure 2b - SEM of the upper surface of the cartilage below
the separated surface layer (x 2200). The surface is
also composed of collagen fibrils, but no broken fibrils are
visible.
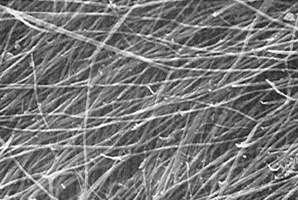
Figure 3a - High-magnification SEM of the deep surface of
the separated superficial layer (x 9000). The fibrils
range in diameter from 75 to 100 nm.
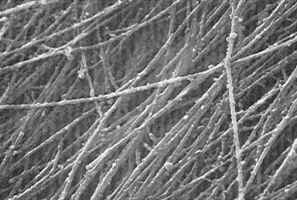
Figure 3b - High-magnification SEM of the surface of the cartilage
portion (x 9000). The fibrils of the surface range from
125 to 150 nm in diameter. A large amount of granular
substance is observed on the fibrils
Macerated specimens. The boundary between the layers
was seen much more clearly in the macerated cartilage specimens
than the fresh specimens on polarising microscopy. The
superficial layer was more easily separated, although no portions
were as larged as those separated from the non-macerated
cartilage. SEM showed a distinct boundary between the superficial
layer and the next layer (fig. 4).
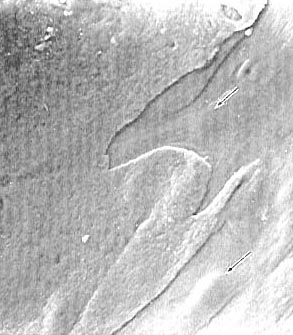
Figure 4 - SEM of the surface of a macerated specimen of articular
cartilage with a partially separated surface layer ( X 330). There
is a distinct boundary between the intact surface on the left
and the surface of the deeper layer on the right. The
gently-sloping elevations (arrows) appear to corespond to
the spindle cells of the deeper layer.
DISCUSSION
MacConaill (1951) reported the existence of the lamina splendens,a
specific superficial structure. A subsequent phase-contrast
microscopic study by Aspden and Hukins (1979) led to the conclusion
that it was an artifact and questioned its existence. Dunharn
et al (1988) confirmed the presence of the lamina splendens
by polarising microscopy. Many transmission electron-microscopic
studies have indicated that the most superficial layer is
composed of collagen fibrils (Weiss, Rosenberg and Helfet
1968; Meachim and Roy 1969; Ghadially 1983; Schenk, Eggli
and Hunziker 1986) and that it is morphologically distinct
from the next deeper layer (Bullough and Goodfellow 1968;
Weiss et al 1968; Wolf 1969).
Meachim and Roy (1969), Ghadially (1983) and Schenk et al
(1986) considered, however, that the most superficial layer
could not be differentiated from the layer deep to it.
Recent SEM observations have confirmed the presence o layer
corresponding to the lamina splendens (Clark 1985 1990; Jeffery
et al 1991). Clark (1990) described the lamina splendens
as bein. 5 my thick and composed of collagen fibrils,
but Jeffery et al (1991) reported it to be an amorphous layer
devoid of collagen fibrils.
In our study, the most superficial layer of the articular
cartilage was seen to be acellular, and composed of collagen
fibrils with a course parallel to the articular surface. The
layer was distinctly different from the next deep layer of
spindle chondrocytes in terms of its optical characteristics
on polarising microscopy and the density and orientation of
collagen fibrils on SEM. The thickness of the superficial
layer was consistently 4 to 8 my regardless the method of
preparation or study. These observations are in close
agreement with those obtained by other authors, using a variety
of methods of observation (Weiss et al 1968; Dunham et al
1988; Clark 1990). All these findings suggest the existence
of an independent acellular superficial layer composed
of collagen fibrils.
If this layer is an independent one with a different collagen
fibril architecture, its boundary surface should be dynamically
fragile and physically separable, as has been previously reported
(Bullough and Goodfellow 1968; Wolf 1969; Clarke 1971). There
have, however, been no previous morphological studies of the
boundary surface at the level of separation.
In our study, the separated specimens of the most superficial
layer showed morphological features identical to those of
untreated specimens. No broken fibrils were seen on SEM
of the inferior surface of any separated portion or on the
surface of any cartilage portion with separated superficial
layer. Specimens macerated with sodium hydroxide showed
the boundary between the superficial and inferior layers much
more clearly.
Our findings confirm that there is a specific lamina splendens
on the articular catilage. It is an acellular, independent
layer of collacen fibrils which is only loosely connected
to the fibrous structure in the deeper layer.
Separation in continuity with the synovium of the neck showed
that the most superficial layer is firmly connected to the
deeper cartilage matrix only in the vicinity of the synovium
and that it undergoes transition to synovial tissue.
Our findinas indicate that the most superficial layer is anchored
firmly in the peripheral region of the articular cartilage
and to the synovial tissue and that its orientation tends
to resist tensile stress durin. joint movement. It seems
that it functions as an outer coating which maintains the
orphological integrity of the articular cartilage by withstanding
extrinsic compression and intrinsic swelling pressure.
No benefits in any form have been received or will be received
from a commercial party related directly or indirectly to
the subject of this article.
REFERENCES
Aspden RM, Hukins DW. The lamina splendens of articular
cartilage is an artefact of phase contrast microscopy. Proc
R Soc Lotid Biol 1979;206:109-13.
Bullough P, Goodfellow J. The significance of the fine structure
of articular cartilage. J Bone Joint Surg [Br] 1968;50-B:852-7.
Clark JM. The organisation of collagen in cryofractured
rabbit articular cartilage: a scanning electron microscopic
study. J Orthop Res 1985;3:17-29.
Clark JM. The organisation of collagen fibrils in the
superficial zones of articular cartilage. J Anat 1990; 171:117-30.
Clarke IC. Articular cartilage: a review and scanning
electron microscope study. 1. The interterritorial fibrillar
architecture. J Bone Joint Surg [Br1 1971;53-B:732-50.
Dunham J, Shackleton DR, Billingham ME, et al. A reappraisal
of the structure of normal canine articular cartilage. J
Anat 1988:157:89-99.
Ghadially FN. Fine structure of synovial joints. London,
etc: Butterworths 1983:80-102.
Jeffery AK, Blunn GW, Archer CW, Bentley G. Three-dimensional
collagen architecture in bovine articular cartilage. J Bone
Joint Surg [Br] 1991:73-B:795-801.
MacConaill MA. The movements of bones and joints: 4.
The mechanical structure of articulating cartilage. J
Bone Joint Surg [Br] 1951;33-B:251-7.
Meachim G, Roy S. Surface ultrastructure of mature adult
human articular cartilage. J Bone Joint Surg [Br]
1969;51-B:529-39.
Schenk RK, Eggli PS, Hunziker EB. Articular cartilage
morphology. In: Kuettner KE, Schleyerbach R, Hascall
VC, eds. Articular cartilage biochemistry. New York,
etc: Raven Press, 1986;3-22.
Weiss C, Rosenberg L, Helfet AJ. An ultrastructural
study of normal young adult human articular cartilage. J
Bone Joint Surg [Am] 1968;50-A:663-74.
Wolf J. Chondrosynovial membrane serving as joint cavity
lining with a sliding and barrier function. Folia Morphol
Praha 1969;17:291-308. |

